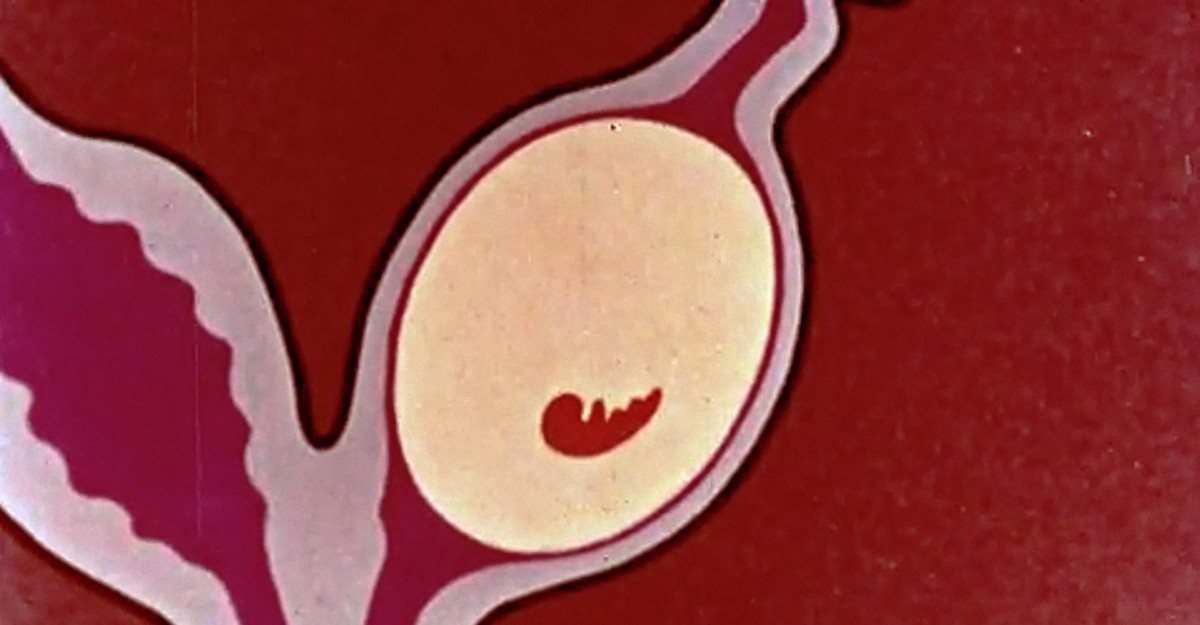
Female tammar wallabies are rarely, if ever, truly alone. Their pregnancies last almost exactly 12 months—and within hours of giving birth, most of the marsupials can be found mating again, conceiving another embryo that they may end up carrying for the next year, save for the single day on which they labor, deliver, and couple up once more.
Bizarrely, most of the embryo’s long stint in utero is spent barely doing anything at all. Once it reaches an 80-cell state, the approximate width of two strands of hair, it arrests its growth and, for 11 months, “just floats,” says Jane Fenelon, a reproductive biologist at the University of Melbourne. It’s a baby in developmental dormancy, a pregnancy that its mother has put on pause.
For most mammals, humans among them, fertilization starts a regimented countdown toward birth. But at least 130 species have found ways to temporarily freeze their gestational clock and delay the most grueling parts of gestation, birth, and lactation until “an optimal time,” says Nucharin Songsasen, a reproductive biologist at the Smithsonian’s Conservation Biology Institute. These animals can sync up their offspring’s arrival with the seasons that will provide the most food; they can conserve their own energy and avoid overspending on ill-timed births. They can even keep an embryo on hold in case an already born offspring dies, revving up a new pregnancy without having to mate again. The bodies of these mothers-to-be effectively turn gestation on and off—in a way, granting themselves a modicum of control over not just how much to invest in kids, but when.
Scientists still don’t know exactly how this phenomenon, called embryonic diapause, works, how common it is, or when or how many times it evolved—but they’re trying to find out. Fitting together those pieces wouldn’t just mean solving one of the biggest puzzles in reproduction. It could change the way that researchers approach species conservation; it could aid the development of new assisted reproductive technology in humans. It may even someday revolutionize the treatment of cancer—a disease that can thwart powerful therapies by entering a stasis of its own.
The prime directive of any mammalian embryo is, simply, to grow. In just days, weeks, or months, one cell must become billions or trillions, a frenetic developmental race that is “really a force of nature,” says Hannele Ruohola-Baker, a biochemist at the University of Washington. “It’s internally controlled, that the embryo will develop, will continue, doesn’t stop.”
At least, that’s usually how it goes. In the mid-1800s, hunters in Europe discovered that female roe deer spotted mating in the summer didn’t have visible embryos in their abdomens until December—a baffling delay. One researcher chalked it up to stunted development; another figured that the deer’s summer trysts had been some sort of reproductive feint, and the real mating was happening secretly in the fall.
Both of those notions were wrong. Roe deer, the scientific community eventually confirmed, were conceiving in summer. But just a couple of days after fertilization, their embryos would slow their growth to a near halt for four or five months, punting birth to the next spring. Evolutionarily, the delay did make sense for all parties involved: Does could mate during the rut, but wouldn’t have to find the calories needed for lactation until food became abundant again; meanwhile, the offspring in the abdomen could wait in an early, low-maintenance state until the world turned hospitable once more.
The discovery shocked scientists, and turned out to be not at all unique. In the decades that followed, a whole menagerie of mammals—among them, badgers, otters, armadillos, bats, and seals—were found to reproduce with similar delays. The lengths, cues, and even frequency of pauses differ so much among species that the trait likely evolved independently more than once, says Jeeyeon Cha, a reproductive biologist at Vanderbilt University Medical Center. Some diapauses, such as those of mice, last mere days and are triggered only if the mother is lactating to feed offspring that have already been born; others, such as those of the American mink, stretch on for weeks, and are cued by the seasonal ebb and flow of daylight. Female black bears, which ovulate repeatedly during breeding season, use diapause to mate with several males—then deliver cubs that share a birthday, but not a dad. And tammar wallabies use both suckling and sunlight to tune their pregnancy dials, coordinating their schedules so that nearly all births occur in late January. The point is for joeys to remain in the pouch for the next eight or nine months, until the Southern Hemisphere’s spring, says Marilyn Renfree, a reproductive biologist at the University of Melbourne: When researchers have chauffeured the marsupials across the equator, the birthing schedules flip.
Even after a century, mammalian diapause feels “counterintuitive” to the ways in which scientists conceive of cellular growth, says Hao Zhu, a cancer biologist at UT Southwestern Medical Center. Cells should no more be able to arrest their metabolism and growth than a human can stop breathing or digesting and still expect to survive. Researchers don’t have a solid sense of how embryos endure the ordeal for so long. “Normally, if you stop cells growing, they die,” Fenelon told me. For now, it seems as though paused embryos are able to hover on the very edge of life—synthesizing only a small number of proteins and running their metabolic motors on low. They ramp down their oxygen use, and pivot from digesting sugars to breaking down their internal stores of fat. “It’s similar to fasting,” says Aydan Bulut-Karslioglu, a biologist at the Max Planck Institute for Molecular Genetics.
At least some embryos can maintain their quiescence for a bafflingly long time: Decades ago, a group of researchers was able to induce a pregnant tammar wallaby into embryonic diapause for more than two years—and when they lifted the hold, her embryo was still able to awaken and come to full term. Even so, Bulut-Karslioglu told me, a theoretical limit for every species must exist. Eventually, scientists have found, the embryos will start to cannibalize their own innards, and may ultimately begin to run out of fuel. And the longer they remain in diapause, she told me, the longer it seems to take the cells to rouse.
Researchers are getting closer to re-creating diapause with embryos in a laboratory dish. But although some scientists, including Bulut-Karslioglu, have come close to perfect mimicry (using mouse embryos), “we are probably missing something,” she told me. The mystery ingredient won’t be simple. For years, researchers hoped that there would be a master chemical or genetic switch that “turned the embryo off,” Fenelon told me. “That doesn’t seem to be the case.” Rather, diapause seems to involve an intermittent dialogue, with the uterus rebuffing the embryo’s attempts to implant until the time is right. For all its quirks, though, the system seems powerful enough to transcend evolutionary barriers: When researchers transplant embryos from sheep (which have standard, unpauseable pregnancies) into the uteruses of mice, the sheep embryos will enter stasis—and then safely resume their development upon returning to a species-appropriate womb.
Scientists still aren’t sure of the signals that thrust certain species into or out of diapause. And answering those questions is only getting more urgent as climate change continues to warp the seasons, says Helen Bateman-Jackson, a wildlife biologist at the Toronto Zoo. Weddell seals, for example, already have to mate or give birth in very narrow windows of time for their pups to survive—a schedule they manage via a brief pregnancy pause, Michelle Shero, a reproductive biologist at the Woods Hole Oceanographic Institution, told me. If rising temperatures or melting ice disrupt the signals that control that halt, their offspring may be more likely to die.
If humans could re-create diapause in labs, scientists could help endangered zoo animals reproduce; hopeful human parents could turn to diapause as an alternative to freezing embryos. Someday, doctors might be able to help their patients better time childbirth to certain life events, or to meet the medical needs of a parent or fetus. Or perhaps diapause-inspired technologies could eventually yield a twist on birth control, Renfree told me, allowing people to effectively halt the reproductive cycle at essentially no cost—like “the best contraceptive you can imagine.”
Inhibiting diapause could also take life away from cells that are causing harm. Certain cancer patients repeatedly find their disease coming back, despite multiple rounds of harsh treatments such as chemotherapy. Researchers used to think that a genetically distinct population of tumor cells were somehow surviving the cull—a problem that could potentially be solved with a different flavor of drug. But in recent years, they have realized that cancer cells may instead be escaping the blitz by pausing their own growth—an eerie parallel to the stasis embryos enter when their mother’s body experiences outside stress. It works because “chemotherapy targets dividing cells,” says Catherine O’Brien, a cancer biologist at the University of Toronto. If cancer cells manage to pause that process, they’ll squeak right on by.
Jinsong Liu, a cancer biologist at the MD Anderson Cancer Center who’s been studying the connection between embryonic diapause and cancer diapause for years, told me that embryos and tumors have a lot in common: Both just desperately want to grow. But those similarities also mean that there could be a way to foil cancer cells’ subterfuge. Researchers could design drugs to block cells from going into stasis, or cook up new therapies that target cells specifically in their paused state. Still other treatments might rewire cells already in dormancy, so that they reactivate as benign entities, rather than tumorous growths ready to invade again. Some of these treatments could be on the market within just a few years—though a clearer sense of how diapause works in both reproductive and cancerous contexts would be key to finagling the therapies just right. Diapause may sometimes delay the genesis of life. But, carefully harnessed, it could someday help postpone the march toward a too-early death.
"like this" - Google News
March 06, 2023 at 07:30PM
https://ift.tt/vS1sbXj
A quirk in animal pregnancy could help humans kill cancer cells - The Atlantic
"like this" - Google News
https://ift.tt/ckT0YFI
Shoes Man Tutorial
Pos News Update
Meme Update
Korean Entertainment News
Japan News Update
No comments:
Post a Comment